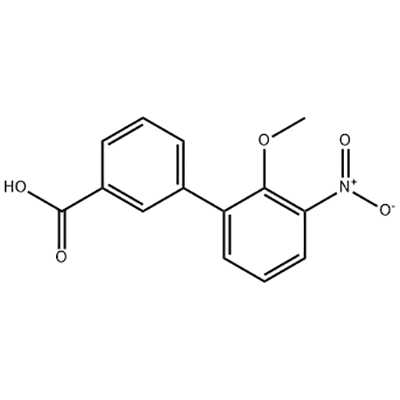
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid
2'-Methoxy-3'-nitro-biphenyl-3-carboxylic acid amagwiritsidwa ntchito ngati wapakatikati wa Eltrombopag.
Eltrombopag, yopangidwa ndi GlaxoSmithKline (GSK) ku UK ndipo pambuyo pake idapangidwa limodzi ndi Novartis ku Switzerland, ndiye woyamba komanso wovomerezeka wa agonist ang'onoang'ono omwe si peptide TPO receptor agonist padziko lapansi.Eltrombopag idavomerezedwa ndi US FDA mu 2008 pochiza idiopathic thrombocytopenic purpura (ITP), komanso mu 2014 pochiza aplastic anemia (AA).Ndiwonso mankhwala oyamba ovomerezedwa ndi US FDA pochiza AA m'zaka 30 zaposachedwa.
Mu december2012, US FDA inavomereza Eltrombopag zochizira thrombocytopenia odwala matenda a chiwindi C (CHC), kuti odwala matenda a chiwindi C ndi osauka matenda chifukwa otsika kupatsidwa zinthu za m`mwazi kuwerenga akhoza kuyamba ndi kusunga interferon zochokera muyezo mankhwala kwa matenda a chiwindi.Pa february3, 2014, GlaxoSmithKline adalengeza kuti FDA idapereka chithandizo chamankhwala chopambana cha Eltrombopag pochiza hemopenia kwa odwala omwe ali ndi chemicalbook aplastic anemia (SAA) omwe sanayankhe mokwanira ku immunotherapy.Pa Ogasiti 24, 2015, US FDA idavomereza Eltrombopag kuti azichiza thrombocytopenia mwa akulu ndi ana azaka za 1 ndi kupitilira omwe ali ndi matenda osachiritsika a immune thrombocytopenia (ITP) omwe alibe yankho lokwanira ku corticosteroids, immunoglobulins kapena splenectomy.Pa january4, 2018, Eltrombopag idavomerezedwa kuti ilembedwe ku China kuti ichiritse primary immune thrombocytopenia (ITP).



![pentamethylene bis [1-(3,4-dimethoxybenzyl) -3,4-dihydro-6,7-dimethoxy-1H-isoquinoline-2-propionate], dioxalate](http://cdn.globalso.com/jindunchem-med/image281-300x300.png)

![Casp ungin Acetate;Caspofungin acetate;Cancidas;Caspofungin acetate [USAN:BAN:JAN];](http://cdn.globalso.com/jindunchem-med/fbe17385-300x300.jpg)