Factory Supply 4-Bromo-2-Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7
Factory Supply 4-Bromo-2-Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7
Kuti tikwaniritse kukhutitsidwa kwamakasitomala komwe timayembekezera, tili ndi gulu lathu lamphamvu lomwe limapereka thandizo lathu lalikulu kwambiri lomwe limaphatikizapo kutsatsa, kugulitsa kwakukulu, kukonzekera, kupanga, kuwongolera kwapamwamba kwambiri, kulongedza, kusungirako katundu ndi katundu wa Factory Supply 4-Bromo-2 -Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7, Ndife oona mtima ndi otseguka.Tikuyembekezera kudzacheza kwanu ndikukhazikitsa ubale wodalirika komanso wanthawi yayitali.
Kuti tikwaniritse kukhutitsidwa kwamakasitomala, tili ndi gulu lathu lamphamvu lomwe limapereka chithandizo chathu chachikulu chomwe chimaphatikizapo kutsatsa, kugulitsa kwakukulu, kukonza mapulani, kupanga, kuwongolera kwapamwamba, kulongedza, kusungirako katundu ndi katunduChina CAS 112704-79-7 ndi 112704-79-7, Katundu wathu wapambana mbiri yabwino kumayiko onse okhudzana.Chifukwa kukhazikitsidwa kwa kampani yathu.Takhala tikuumirira pakupanga njira zatsopano zopangira zinthu pamodzi ndi njira zamakono zoyang'anira masiku ano, kukopa anthu ambiri omwe ali ndi luso pamakampani awa.Timawona yankho labwino ngati umunthu wathu wofunikira kwambiri.
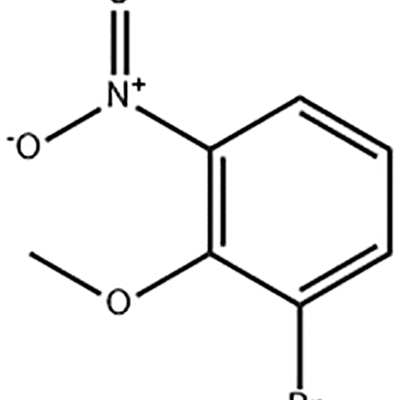
1-Bromo-2-methoxy-3-nitro-benzene amagwiritsidwa ntchito ngati wapakatikati wa Eltrombopag.
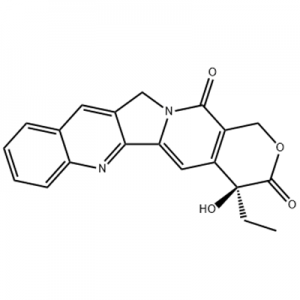
Eltrombopag, yopangidwa ndi GlaxoSmithKline (GSK) ku UK ndipo pambuyo pake idapangidwa limodzi ndi Novartis ku Switzerland, ndiye woyamba komanso wovomerezeka wa agonist ang'onoang'ono omwe si peptide TPO receptor agonist padziko lapansi.Eltrombopag idavomerezedwa ndi US FDA mu 2008 pochiza idiopathic thrombocytopenic purpura (ITP), komanso mu 2014 pochiza aplastic anemia (AA).Ndiwonso mankhwala oyamba ovomerezedwa ndi US FDA pochiza AA m'zaka 30 zaposachedwa.
Mu december2012, US FDA inavomereza Eltrombopag zochizira thrombocytopenia odwala matenda a chiwindi C (CHC), kuti odwala matenda a chiwindi C ndi osauka matenda chifukwa otsika kupatsidwa zinthu za m`mwazi kuwerenga akhoza kuyamba ndi kusunga interferon zochokera muyezo mankhwala kwa matenda a chiwindi.Pa february3, 2014, GlaxoSmithKline adalengeza kuti FDA idapereka chithandizo chamankhwala chopambana cha Eltrombopag pochiza hemopenia kwa odwala omwe ali ndi chemicalbook aplastic anemia (SAA) omwe sanayankhe mokwanira ku immunotherapy.Pa Ogasiti 24, 2015, US FDA idavomereza Eltrombopag kuti azichiza thrombocytopenia mwa akulu ndi ana azaka za 1 ndi kupitilira omwe ali ndi matenda osachiritsika a immune thrombocytopenia (ITP) omwe alibe yankho lokwanira ku corticosteroids, immunoglobulins kapena splenectomy.Pa january4, 2018, Eltrombopag idavomerezedwa kuti ilembedwe ku China kuti ichiritse primary immune thrombocytopenia (ITP).
Kuti tikwaniritse kukhutitsidwa kwamakasitomala komwe timayembekezera, tili ndi gulu lathu lamphamvu lomwe limapereka thandizo lathu lalikulu kwambiri lomwe limaphatikizapo kutsatsa, kugulitsa kwakukulu, kukonzekera, kupanga, kuwongolera kwapamwamba kwambiri, kulongedza, kusungirako katundu ndi katundu wa Factory Supply 4-Bromo-2 -Fluorobenzoic Acid 4-Bromo-2-Fluorobenzoic Acid CAS 112704-79-7, Ndife oona mtima ndi otseguka.Tikuyembekezera kudzacheza kwanu ndikukhazikitsa ubale wodalirika komanso wanthawi yayitali.
Factory SupplyChina CAS 112704-79-7 ndi 112704-79-7, Katundu wathu wapambana mbiri yabwino kumayiko onse okhudzana.Chifukwa kukhazikitsidwa kwa kampani yathu.Takhala tikuumirira pakupanga njira zatsopano zopangira zinthu pamodzi ndi njira zamakono zoyang'anira masiku ano, kukopa anthu ambiri omwe ali ndi luso pamakampani awa.Timawona yankho labwino ngati umunthu wathu wofunikira kwambiri.